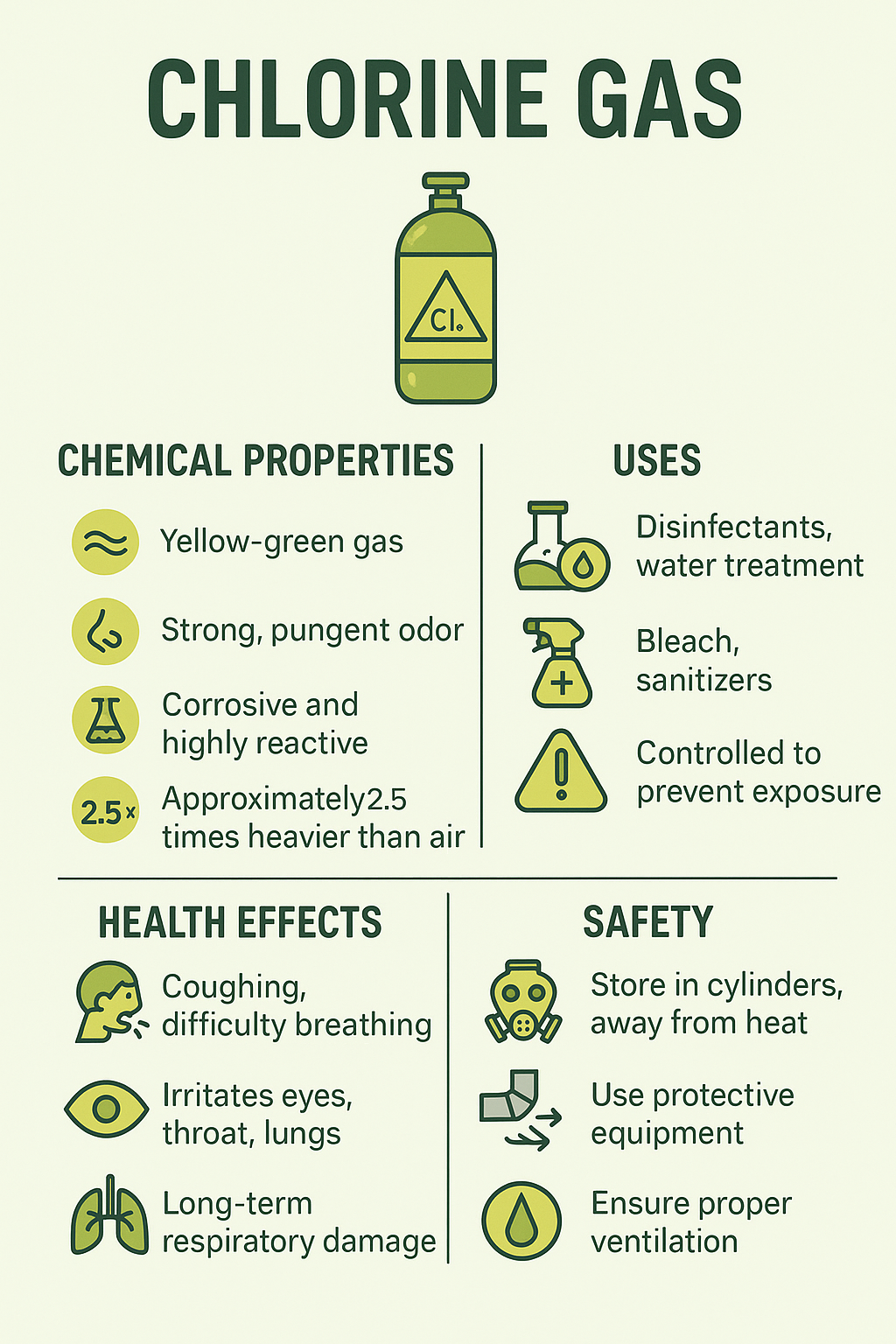
Chlorine gas is a widely used chemical element with significant industrial applications, but it is also a highly toxic and corrosive substance.
Recognised by its distinctive yellow-green colour and strong, irritating odour, chlorine is valued for its disinfecting and bleaching capabilities. At the same time, its reactivity and potential to cause harm make it a hazardous material that must be handled with great care.
From water treatment plants to chemical manufacturing facilities, chlorine’s uses are broad, yet its safe management requires strict control measures, reliable detection systems, and thorough knowledge of its properties.
Chlorine is a chemical element, symbol Cl, and is classified as a halogen in Group 17 of the periodic table. At room temperature, it exists as a diatomic molecule (Cl₂), appearing as a dense, yellow-green gas with a strong, pungent odour that serves as an immediate warning of its presence. Even at low concentrations, chlorine vapour can irritate the eyes, throat, and respiratory tract.
It is approximately 2.5 times heavier than air, which means it tends to accumulate in low-lying areas, creating additional hazards in enclosed or poorly ventilated spaces.
Chlorine is moderately soluble in water, producing a pale yellow solution with a sharp smell. When dissolved, it reacts to form hypochlorous acid and hydrochloric acid — both corrosive and highly reactive compounds.
As a strong oxidising agent, chlorine reacts readily with many elements and compounds. It can combine with metals, non-metals, and organic materials, often releasing heat and potentially harmful fumes.
This reactivity makes it valuable in industrial chemical processes but also demands stringent safety measures during storage, transport, and use. Its toxic and corrosive characteristics mean that direct exposure, even in small amounts, can present serious health risks.
Most industrial chlorine gas is produced through the electrolysis of brine (a concentrated sodium chloride solution). This process involves passing an electric current through brine in specialised equipment, splitting the sodium chloride into chlorine gas, hydrogen gas, and sodium hydroxide. The chlorine produced is then collected, purified, and stored for use in various applications.
Several electrolysis methods are used in industry, including the diaphragm cell, membrane cell, and mercury cell processes. Each method has specific operational requirements and environmental considerations, but all rely on secure handling systems to prevent leaks and exposure.
In addition to deliberate production, chlorine gas can also be released as a by-product of other chemical processes, such as in certain types of paper and textile bleaching, or the manufacture of chlorinated solvents and plastics. Older or poorly maintained equipment in these processes can increase the risk of unplanned releases.
On a smaller scale, chlorine can be generated on-site in facilities such as swimming pools and water treatment plants, often through controlled chemical reactions or compact electrolysis units. While these operations typically involve lower volumes than large-scale industrial production, the handling requirements remain stringent because of the gas’s corrosive, toxic, and reactive properties.
The widespread use of chlorine across industrial, commercial, and public health sectors makes its safe production a matter of both operational efficiency and environmental responsibility.

Chlorine gas has a wide range of industrial, commercial, and public health applications, making it one of the most versatile chemicals in modern manufacturing and sanitation. Its strong oxidising properties and ability to kill bacteria and other harmful microorganisms have made it indispensable in several sectors — though its toxic and corrosive nature means that every use is carefully controlled.
Industrial manufacturing
One of chlorine’s most significant industrial applications is in the production of polyvinyl chloride (PVC), a durable plastic used in pipes, cables, window frames, and countless other products. It is also employed in the manufacture of solvents, synthetic rubbers, and a variety of organic and inorganic chemicals. These processes often require chlorine as a raw material to introduce chlorine atoms into molecular structures, altering properties such as durability, flame resistance, and chemical stability.
● Bleach and disinfectant production
Chlorine is a key ingredient in the manufacture of bleach and other cleaning agents. In its various forms, it is used as a disinfectant and sanitiser for surfaces in homes, hospitals, and food processing facilities. These products work by breaking down the cell walls of microorganisms, effectively neutralising bacteria, viruses, and fungi.
● Water and wastewater treatment
For decades, chlorine has been the most widely used chemical for disinfecting drinking water and treating wastewater. By controlling harmful pathogens, it has played a major role in reducing the spread of waterborne diseases. Municipal water systems often use gaseous chlorine or hypochlorite solutions, with precise dosing to balance effective disinfection against the risks of overexposure.
● Swimming pool maintenance
In recreational settings, chlorine is used to maintain safe water quality in swimming pools and spas. It helps prevent the growth of algae and controls bacteria, keeping water clear and hygienic. While most pool treatment products rely on chlorine compounds rather than pure chlorine gas, large facilities may use gas-fed systems for efficiency.
● Other specialised applications
Chlorine is also used in the production of pesticides, pharmaceuticals, and certain dyes. In controlled environments, it can be applied in metallurgy for refining metals or in the electronics industry for etching circuits.
Each of these uses demands careful engineering controls and trained personnel to manage the risks associated with chlorine’s reactivity and toxicity.
Chlorine gas is toxic to humans, and exposure can be harmful even at relatively low concentrations. Its strong oxidising and corrosive properties mean that it readily reacts with moisture in the eyes, throat, and respiratory tract, producing acids that damage tissue. The severity of health effects depends on the concentration of the gas, the duration of exposure, and the individual’s level of sensitivity.
● Acute effects
Inhalation of chlorine gas, even in small amounts, can cause immediate irritation of the nose, throat, and lungs. Early symptoms may include coughing, shortness of breath, chest tightness, and a burning sensation in the eyes and throat. At higher concentrations, exposure can lead to severe respiratory distress, wheezing, and fluid accumulation in the lungs (pulmonary oedema), which can be life-threatening. Eye contact can result in redness, tearing, and temporary vision disturbance, while skin contact may cause redness, itching, or blistering.
● Chronic exposure risks
Prolonged or repeated low-level exposure, often a risk in certain industrial workplaces without adequate safety measures, can lead to long-term respiratory conditions. These include chronic bronchitis, asthma-like symptoms, and reduced lung function. Repeated skin contact can also cause dermatitis due to the gas’s corrosive nature. Dental erosion has been reported in some workers who experience ongoing exposure to chlorine vapours.
● Vulnerable populations
Individuals with existing respiratory issues, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly susceptible to chlorine’s effects. Children and older adults may also be more affected due to differences in lung capacity and immune response.
Because of these hazards, strict workplace safety protocols and regular health monitoring are necessary in environments where chlorine is present. Immediate medical attention is recommended after any significant exposure, regardless of symptom severity, as some respiratory effects can be delayed.
Chlorine gas, due to its toxic and corrosive nature, can have significant effects on the environment when released. Its impact is most apparent in air and water, where it can cause both immediate and long-term harm to living organisms.
● Air quality
In the atmosphere, chlorine gas acts as an irritant to both humans and wildlife. Short-term exposure to elevated concentrations can harm birds and small mammals, whose respiratory systems are particularly sensitive. While chlorine does not persist in the air for extended periods — reacting quickly with moisture and other compounds — the by-products of these reactions can still be hazardous.
● Water pollution
When chlorine enters rivers, lakes, or other water bodies, it dissolves and reacts with organic matter to form hypochlorous acid and hypochlorite ions. These compounds are effective disinfectants but can also be harmful to aquatic life, damaging fish gills, impairing reproduction, and affecting invertebrate populations. The reaction between chlorine and naturally occurring organic matter can produce disinfection by-products such as trihalomethanes, which are toxic to both humans and wildlife at high levels.
● Soil and vegetation
Chlorine exposure can damage plant tissue, causing leaf burn, reduced growth, and in some cases, plant death. In agricultural or natural environments, accidental releases can have lasting effects on crops and surrounding vegetation.
Because chlorine is highly reactive, it does not typically accumulate in the environment. However, its immediate effects on ecosystems can be severe, making prevention of accidental releases a priority in any facility where the gas is produced, stored, or used.
Working safely with chlorine gas requires strict controls, well-trained personnel, and the right protective systems in place. Because the gas is toxic, corrosive, and highly reactive, handling procedures are designed to prevent leaks, minimise exposure, and protect both people and the environment.
● Storage requirements
Chlorine gas is typically stored in specially designed steel cylinders or larger bulk tanks. These containers must be kept in cool, dry, and well-ventilated areas, away from direct sunlight and incompatible substances such as ammonia, hydrogen, and hydrocarbons. Storage facilities should be fitted with leak detection equipment and secondary containment systems to reduce the risk of accidental release.
● Safe handling practices
Only trained and authorised personnel should handle chlorine cylinders or equipment. Handling should be done in areas with adequate ventilation or local exhaust systems to prevent gas build-up. Care must be taken when connecting or disconnecting cylinders, using approved tools and fittings to avoid damage to valves or seals.
● Personal protective equipment (PPE)
Workers should wear suitable PPE, which may include chemical-resistant gloves, safety goggles or face shields, and protective clothing. In situations where airborne concentrations could exceed safe limits, respiratory protection such as full-face respirators or self-contained breathing apparatus (SCBA) is required.
● Routine maintenance and inspections
Regular inspection of storage containers, pipelines, and control systems is essential. Preventive maintenance helps detect wear or corrosion before it can lead to leaks. Instruments for monitoring chlorine concentration should also be calibrated and tested according to manufacturer recommendations.
● Training and awareness
Staff should receive ongoing training covering the hazards of chlorine gas, safe handling techniques, emergency response, and the proper use of detection and protective equipment.
Maintaining a strong safety culture is critical to preventing incidents.
Chlorine gas is a highly reactive substance, capable of forming hazardous compounds when it comes into contact with certain materials. Its strong oxidising ability means that even small leaks can create dangerous chemical reactions if incompatible substances are present.
● Reactions with other chemicals
Chlorine reacts violently with ammonia, producing toxic chloramine vapours that can cause severe respiratory irritation. In the presence of excess ammonia, nitrogen trichloride can form — a highly explosive compound. It also reacts with hydrogen under certain conditions to form hydrogen chloride, which is corrosive and irritating to the respiratory system. With hydrocarbons, chlorine can produce chlorinated organic compounds, some of which are harmful or environmentally persistent.
● Potential for combustion and explosions
While chlorine itself is not flammable, it can support combustion in other materials. For example, it can react with finely divided metals such as aluminium, copper, and iron, releasing heat and creating a fire risk. In some cases, the heat generated by these reactions is enough to ignite combustible materials.
● Industrial risk factors
Facilities that use chlorine alongside other reactive chemicals — such as fertiliser plants, paper mills, or water treatment works — must take extra precautions to prevent cross-contamination. This includes using dedicated pipelines, maintaining separation of storage areas, and implementing strict control measures during transfer or mixing operations.
● Corrosive effects
Chlorine’s reaction with moisture to form hydrochloric and hypochlorous acids contributes to its corrosive impact on metals and equipment. This makes proper material selection for pipelines, seals, and fittings critical in preventing leaks or equipment failure.
Because chlorine gas is toxic even at low concentrations, effective detection and monitoring systems are a critical part of workplace safety. Early warning is essential to protect personnel, limit environmental release, and allow for rapid corrective action.
Portable detection equipment
Handheld portable chlorine gas detectors are commonly used for routine inspections, leak checks, and personal safety monitoring. These devices often use electrochemical sensors to detect chlorine in parts per million (ppm), providing real-time readings and alarms. Portable units are especially valuable for maintenance crews or workers moving between different areas, as they can identify localised hazards before they become widespread.
Fixed monitoring systems
Fixed chlorine gas detectors are installed in key locations where the gas is stored, used, or could accumulate. These systems continuously monitor the air, triggering visual and audible alarms when chlorine concentrations exceed pre-set limits. They can also be integrated into ventilation or emergency shutdown systems for automated responses to leaks.
Detection methods
Electrochemical sensors are widely used for their accuracy and sensitivity, but other methods such as colorimetric detection tubes can also provide spot measurements. In high-risk environments, redundant detection methods may be employed for added reliability.
Placement and calibration
Because chlorine is heavier than air, detectors should be placed near the floor or in low-lying areas where the gas is likely to settle. Regular calibration and maintenance of detection equipment are essential to maintain performance and accuracy.
These solutions are designed to provide reliable monitoring, helping facilities meet safety standards and protect workers from chlorine exposure.
A chlorine leak can escalate quickly, so having clear emergency response procedures is critical for protecting people, property, and the environment. Because the gas is toxic, corrosive, and heavier than air, it can spread rapidly in low-lying areas, making early detection and swift action essential.
Immediate actions
At the first sign of a leak — whether detected by monitoring equipment, visual observation, or odour — workers should raise the alarm and evacuate the area. Only trained emergency response teams equipped with the proper protective equipment, such as self-contained breathing apparatus (SCBA), should attempt to investigate or contain the leak.
● Isolation and containment
If safe to do so, the source of the leak should be isolated by closing valves or shutting down relevant equipment. Ventilation systems may be activated to disperse the gas, but care must be taken to avoid directing it into occupied areas. In some cases, water spray curtains are used to help absorb chlorine vapours and limit their spread.
● Evacuation procedures
Workers should be evacuated to a designated safe zone upwind from the release site. Because chlorine is heavier than air, it may collect in trenches, pits, or basements, so routes should avoid these areas. Emergency coordinators should account for all personnel before allowing re-entry.
● First aid for exposure
Inhalation exposure requires moving the affected person to fresh air immediately, keeping them at rest, and seeking urgent medical attention. If breathing is difficult, trained personnel may provide oxygen. Eye or skin contact should be treated by flushing with plenty of water for at least 15 minutes, followed by prompt medical evaluation.
● Coordination with emergency services
Major leaks may require assistance from local fire brigades or hazardous materials (HAZMAT) teams. Facilities should maintain up-to-date contact information and be prepared to share details about the quantity of chlorine on-site, storage locations, and site layout.
The handling, storage, and transport of chlorine gas are regulated to protect workers, the public, and the environment. In Ireland and across the EU, chlorine is classified as a hazardous substance under chemical safety legislation, meaning that its use is subject to strict safety standards and regulatory compliance requirements.
● EU regulatory framework
Chlorine gas is covered under the EU CLP Regulation (Classification, Labelling and Packaging of Substances and Mixtures), which sets hazard classifications, labelling requirements, and safety data sheet standards. It is also regulated by the REACH Regulation (Registration, Evaluation, Authorisation and Restriction of Chemicals), requiring manufacturers and importers to register and provide safety information on its use.
● Workplace safety requirements
In Ireland, the Health and Safety Authority (HSA) enforces regulations on the safe use of hazardous chemicals, including chlorine. Employers must carry out risk assessments, provide training, and implement control measures to prevent harmful exposure. This includes the use of engineering controls, personal protective equipment, and emergency procedures in line with the Safety, Health and Welfare at Work (Chemical Agents) Regulations.
● Seveso III Directive
Chlorine is listed under the EU’s Seveso III Directive for the control of major accident hazards involving dangerous substances. Facilities that store chlorine above specified thresholds must prepare a safety report, maintain an on-site emergency plan, and coordinate with local authorities on external emergency planning.
● Transport and storage standards
The carriage of chlorine gas is governed by ADR (European Agreement concerning the International Carriage of Dangerous Goods by Road) regulations. These outline packaging, labelling, and vehicle requirements for safe transport. Storage facilities must meet design and maintenance standards to minimise the risk of accidental release.
Strict adherence to these regulations helps reduce the risks associated with chlorine gas, while ensuring that operations meet both legal obligations and industry best practice.
Who discovered chlorine gas?
Chlorine was first recognised as a distinct chemical element in 1774 by Swedish chemist Carl Wilhelm Scheele. He produced it by reacting hydrochloric acid with manganese dioxide, although he initially thought it was a compound rather than an element. It was later identified as an element by Sir Humphry Davy in 1810, who named it “chlorine” after the Greek word chloros, meaning greenish-yellow.
Is chloride the same as chlorine?
No. Chlorine is a chemical element in its pure form, typically found as a yellow-green gas at room temperature. Chloride refers to the negatively charged ion (Cl⁻) formed when chlorine gains an electron, usually in a compound such as sodium chloride (table salt). Chloride is stable and non-toxic at normal levels in the body, whereas chlorine gas is highly reactive and toxic.
Is chlorine gas harmful to humans?
Yes. Inhalation, skin contact, or eye exposure can cause harmful effects ranging from mild irritation to severe respiratory distress. Prolonged or high-level exposure can lead to chronic health problems and, in extreme cases, can be fatal.
What is chloride gas used for?
The term “chloride gas” is not chemically correct, as chloride typically exists in stable compounds rather than as a gas. Chlorine gas, however, is widely used in industry for producing chemicals, disinfectants, bleach, and in water treatment processes.
Managing chlorine gas safely requires the right expertise, reliable equipment, and a clear understanding of regulatory requirements. Whether you operate a water treatment plant, a manufacturing facility, or another environment where chlorine is present, taking proactive measures can protect your team, your operations, and the surrounding community.
OBW Technologies offers a complete range of portable and fixed gas detection solutions, supported by expert advice on safe storage, handling, and emergency preparedness for chlorine gas. We work closely with our clients to assess risks, recommend the right monitoring equipment, and guide them through compliance with Irish and EU regulations.
Whether you need a site assessment, equipment selection, or help meeting safety requirements, our specialists are ready to assist. Contact OBW Technologies to discuss how we can help you strengthen chlorine gas safety in your workplace.
From the Blog



If you have any questions about our products or services, please feel free to contact us.